Module VGAS
Fundamentals
The combustion of a pure gas (e.g. methane) with air takes place according to a defined stoichiometry, which results from the reaction equation. For gas mixtures, a separate reaction equation is required for each component. If the composition of the fuel gas is fixed, the amount of oxygen required for stoichiometric combustion can be determined. By balancing according to the Ideal Gas Law, the amount of air and flue gas is also fixed.
Reaction equations
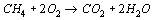
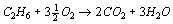
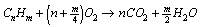
Program features
Combustion equations are independently established from the fuel gas composition specification and the air or flue gas quantity required for stoichiometric combustion is determined. Gas mixtures with up to 10 gas components can be specified. The air number gives the actual combustion air volume and the composition of the flue gas (main components only). Incomplete combustion with air numbers less than 1 is not included.
Operation:
All components (modules) of our system are structured under a uniform user interface. The operation of the different modules is always identical within the ATLAS program system.
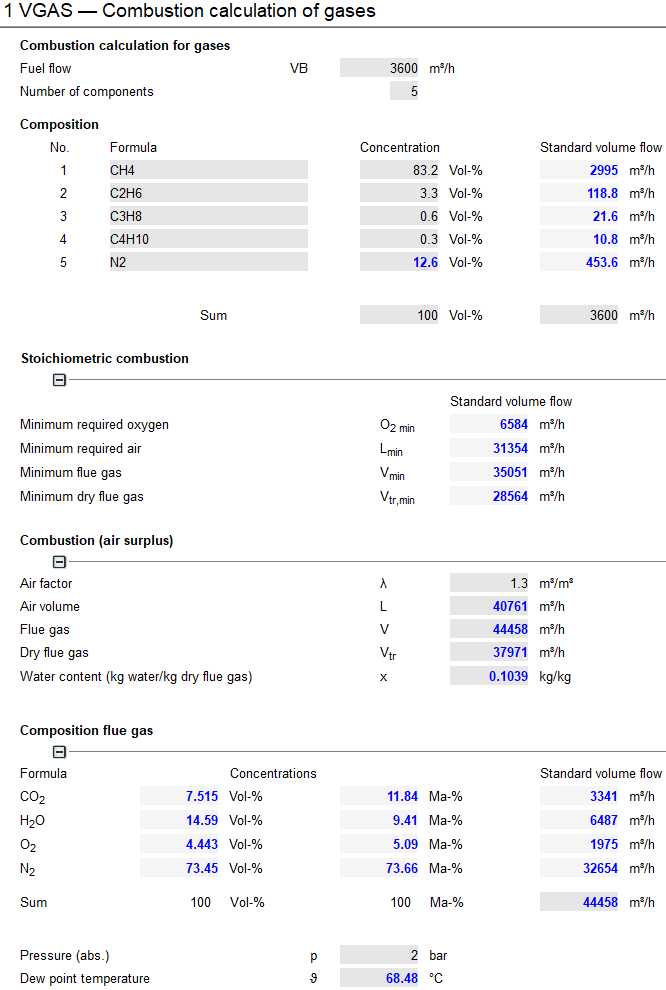 Input mask VGAS
Input mask VGAS
see also:
Dieser Beitrag ist auch verfügbar auf:
 Deutsch (German)
Deutsch (German)